Fuel cells
Fuel Cell- What is it?
A fuel cell is a machine that generates energy by oxidizing fuel in a chemical reaction. Any fuel that can be oxidized, such as hydrogen, methane, propane, methanol, diesel, or gasoline, could be used as the source fuel. If air is employed as the oxidizer, the only by-products are water and a tiny amount of nitrous oxide.
From autos to mobile phones to spacecraft, fuel cells may be used to power almost everything. Bloom Energy introduced Bloom Boxes, a fuel cell product, in February 2010. Bloom Boxes are modular fuel cell constructions that can be used in conjunction to generate the necessary quantity of electricity for a particular purpose. The ordinary home could be powered by a stack of fuel cells approximately the size of a loaf of bread; one fuel cell could power a single lightbulb. Five parking-space-sized Bloom Boxes were supplying 15% of the campus's electricity at the time of the announcement. Coca-Cola, FedEx, Google, and Wal-Mart were among of Bloom's additional beta users. Because the boxes have a modular design akin to data centre servers, Bloom refers to them as "energy servers."
Merits of using fuel cell
Compared to conventional power generation, fuel cells have a number of advantages, including higher fuel efficiency, reduced noise, and zero hazardous emissions at the point of use.
Because they both produce energy, fuel cells and batteries have many similarities. However, a fuel cell uses an external fuel, like hydrogen, allowing it to continue working as long as fuel is available, whereas a battery stores energy in its electrodes. However, unlike typical batteries, fuel cells don't have any moving parts and don't contain any hazardous compounds, which reduces the need for maintenance.
Several other benefits of fuel cells include:
High performance
prompt refuelling
low-key operation
hardly vibrating
Small size
Low maintenance costs and quick reaction to variations in electricity demand
lengthy lifespans.
An electrode and anodes are the same components of all fuel cells, however different types of fuel cells can be distinguished primarily by the electrolyte they employ. PEM (proton exchange membrane), DMFC (direct methanol fuel cell), MCFC (molton carbonate fuel cell), PAFC (phosphoric acid fuel cell), SOFC (solid oxide fuel cell), and AFC are the six primary types of fuel cells (alkaline fuel cell). The proton exchange membrane fuel cell is the leading technology because of its adaptability, toughness, and applicability in a variety of applications.
Single fuel cells are stacked one on top of the other to form a fuel cell "stack". The fuel cell stack may be made up of hundreds of individual cells stacked together depending on the application; this scalability enables fuel cells to be arranged into a broad range of sizes to fit the needed amount of energy, whether it be for a train, drone, building, or car. A traction motor is then propelled by the electricity generated by a fuel cell to move the wheels of a car or any other object you may be powering.
Worldwide, numerous governments are establishing requirements and deadlines for the adoption of electric vehicles with zero emissions. Fuel cells are often viewed as the technology of choice for individuals who want greater vehicle range capabilities, while battery electric vehicles will be perfect as city cars. However, user desire will ultimately determine whether the alternative is battery or fuel cells. Due to the limitations of battery-only choices in terms of weight and range, hydrogen fuel cells are thought to be the most efficient option for heavier vehicles like trucks or transit buses.
How does a fuel cell operate?
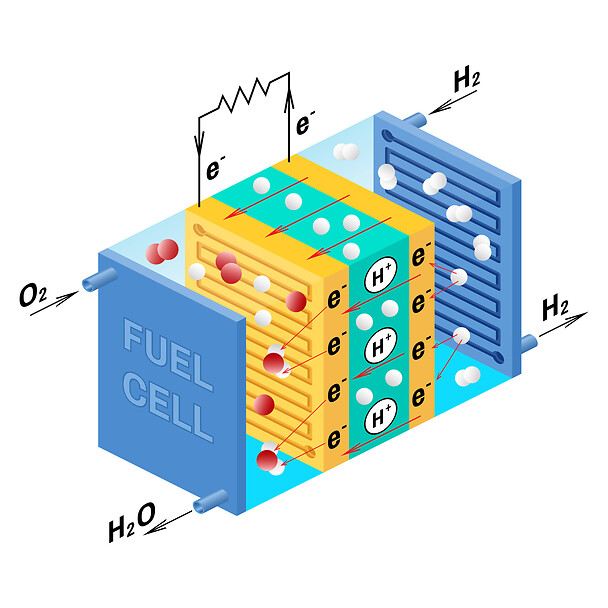
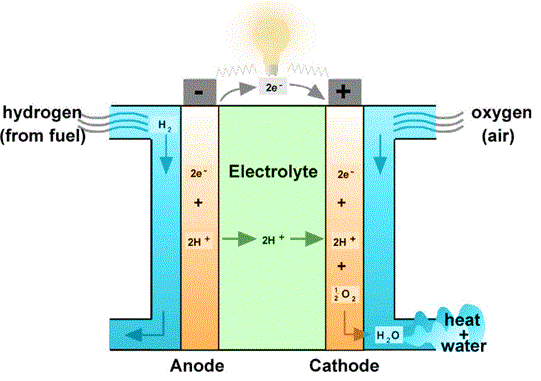
An electrolyte is placed between two electrodes in a single fuel cell. In order to produce electricity, water, and heat, airborne hydrogen and oxygen are passed across two electrodes, respectively. The electrical current is collected and the gases are distributed by bipolar plates on either side of the cell.
Hydrogen is split into ions and electrons at the anode using platinum or a comparable catalyst. While the ions move through the electrolyte to the cathode, where, with the aid of another catalyst, they combine with oxygen atoms to generate water, the electrons move via an open circuit, producing the necessary amount of electricity.
Types of fuel cell
Alkali fuel cell
Compressed hydrogen and oxygen are used to power alkali fuel cells. As their electrolyte, they often use a potassium hydroxide (chemically, KOH) solution in water. Operating temperature is between 150 and 200 degrees C, and efficiency is around 70 percent (about 300 to 400 degrees F). 300 watts (W) to 5 kilowatts (W) are the ranges of cell output (kW). In the Apollo spacecraft, alkali cells were employed to generate electricity and purify water. However, their platinum electrode catalysts are expensive, and they need pure hydrogen fuel. They can also leak, just like any liquid-filled container.
Molten Carbonate
The electrolyte in molten carbonate fuel cells (MCFC) is a high-temperature salt carbonate compound (CO3), such as sodium or magnesium. Operating temperature is roughly 650 degrees C, and efficiency is between 60 and 80 percent (1,200 degrees F). There are plans for units with an output of up to 100 MW, and units with an output as low as 2 MW have already been built. The cell's ability to be "poisoned" by carbon monoxide is limited by the high temperature, and leftover heat can be recycled to generate more energy. Compared to the platinum utilised in other cells, their nickel electrode-catalysts are less expensive. The materials and safe applications of MCFCs are nevertheless constrained by their high temperature; they would likely be too hot for use in homes.
Proton Exchange Membrane
The polymer electrolyte used in proton exchange membrane (PEM) fuel cells take the form of a thin, permeable sheet. Operating temperature is about 80 degrees C, and efficiency is between 40 and 50 percent (about 175 degrees F). The typical range of cell outputs is 50 to 250 kW. These cells function at a low enough temperature to make them suitable for use in homes and automobiles, and the solid, flexible electrolyte will not leak or split. However, their fuels must be cleaned, and the use of a platinum catalyst on both sides of the membrane raises the price.
Fuel cells have a wide range of uses, including producing electricity for transportation, commercial, industrial, and residential structures, as well as long-term energy storage for the grid in reversible circuits.